Somatic fusion
Somatic fusion, also called protoplast fusion, is a type of genetic modification in plants by which two distinct species of plants are fused together to form a new hybrid plant with the characteristics of both, a somatic hybrid. Hybrids have been produced either between the different varieties of the same species (e.g. between non-flowering potato plants and flowering potato plants) or between two different species (e.g. between wheat triticum and rye secale to produce Triticale).
Uses of somatic fusion include making potato plants resistant to potato leaf roll disease.[1] Through somatic fusion, the crop potato plant Solanum tuberosum – the yield of which is severely reduced by a viral disease transmitted on by the aphid vector – is fused with the wild, non-tuber-bearing potato Solanum brevidens, which is resistant to the disease. The resulting hybrid has the chromosomes of both plants and is thus similar to polyploid plants.
Process for plant cells
The somatic fusion process occurs in four steps:[2]
- The removal of the cell wall of one cell of each type of plant using cellulase enzyme to produce a somatic cell called a protoplast
- The cells are then fused using electric shock (electrofusion) or chemical treatment to join the cells and fuse together the nuclei. The resulting fused nucleus is called heterokaryon.
- The cells are then grown into calluses which then are further grown to plantlets and finally to a full plant, known as a somatic hybrid.
Different from the procedure for seed plants describe above, fusion of moss protoplasts can be initiated without electric shock but by the use of polyethylene glycol (PEG). Further, moss protoplasts do not need phytohormones for regeneration, and they do not form a callus.[3] Instead, regenerating moss protoplasts behave like germinating moss spores.[4] Of further note sodium nitrate and calcium ion at high pH can be used, although results are variable depending on the organism.[5]
Methods of somatic hybridization
Procedure for successful somatic hybridization is as below: (i) isolation of protoplasts from suitable plants, (ii) mixing of protoplasts in centrifuge tube containing fugigenic chemicals i.e. chemicals promoting protoplast fusion, such as polyethylene glycol (PEG) (20%, W/V), sodium nitrate (NaNO3), maintenance of high pH 10.5 and temperature 37°C (as a result of fusion of protoplasts viable heterokaryons are produced. PEG induces fusion of plant protoplasts and animal cells and produces heterokaryon (Davey et al, 1978), (iii) wall regeneration by heterokaryotic cells, (iv) fusion of nuclei of heterokaryon to produce hybrid cells, (v) plating and production of colonies of hybrid cells, (vi) selection of hybrid, subculture and induction of organogenesis in the hybrid colonies, and (vii) transfer of mature plants from the regenerated callus.
|
Applications in animal cells
Somatic cells of different types can be fused to obtain hybrid cells. Hybrid cells are useful in a variety of ways, e.g.,
(v) production of monoclonal antibodies by producing hybridoma (hybrid cells between an immortalised cell and an antibody producing lymphocyte), etc.
Male Sterility
Male sterility is defined as an absence or non-function of pollen grain in plant or incapability of plants to produce or release functional pollen grains. The use of male sterility in hybrid seed production has a great importance as it eliminate the process of mechanical emasculation.
Types of Male Sterility:
The male sterility is of five types 1) Genetic male sterility, 2) Cytoplasmic male sterility, 3) Cytoplasmic genetic male sterility, 4) Chemical induced male sterility and 5) Transgenic male sterility.
1) Genetic Male Sterility:
The pollen sterility, which is caused by nuclear genes, is termed as genic or genetic male sterility. It is usually governed by a single recessive gene ms or ‘s’ with monogenic inheritance, but dominant gene governing male sterility are also known E.g Safflower. The male sterility alleles may rise spontaneously or it can be induced artificially and is found in several crops viz. Pigeon pea, castor, tomato, limabean, barley, cotton, etc. A male sterile line may be maintained by crossing it with heterozygous male fertile plant, such a mating produces 1:1 male sterile and male fertile plants.
Utilization in Plant Breeding:
Genetic male sterility is usually recessive and monogenic hence can be used in hybrid seed production. It is used in both seed propagated crops and vegetatively propagated species. In this progeny from crosses ( msms X Msms) are used as a female and are inter planted with homozygous male fertile ( MsMs) pollinator. The genotypes of msms and Msms lines are identical except for the ‘ms’ locus i.e. they are isogenic and are known as male sterile A) Maintainer B) Line respectively. The female line would
Therefore contain both male sterile and male fertile and male fertile plants, the later must be identified and removed before pollen shedding. This is done by identifying the male fertile plants in seeding stage either due to the pleiotrophic effect of ms gene or due to phenotypic effect of closely lined genes.
Therefore contain both male sterile and male fertile and male fertile plants, the later must be identified and removed before pollen shedding. This is done by identifying the male fertile plants in seeding stage either due to the pleiotrophic effect of ms gene or due to phenotypic effect of closely lined genes.
2) Cytoplasmic Male Sterility:
The pollen sterility which is controlled by cytoplasmic genes is known as cytoplasmic male sterility (CMS). Usually the cytoplasm of zygote comes primarily from the eggs cell and due to this progeny of such male sterile plants would always be male sterile.
CMS may be transferred easily to a given strain by using that strain as a pollinator (recurrent parent) in the successive generation of backcross programme. After 6-7 backcrosses the nuclear genotype of male sterile line would be almost identicle to that of the recurrent pollinator strain. The male sterile line is maintained by crossing it with pollinator strain used as a recurrent parent in backcross, since the nuclear genotype of the pollinator is identicle with that of the new male sterile line. Such a male fertile line is known as maintainer line or ‘B’ line and ‘male sterile line is also known as ‘A ‘ line. Cytoplasmic male sterile is not influenced by environmental factor and it resides in maize in mitochondria.
Utilization in Plant Breeding:
CMS has limited application. It cannot be used for development of hybrid, where seed is the economic product. But it can be used for producing hybrid seed in certain ornamental species or asexually propagated species like sugarcane, potato, and forage crops.
3) Cytoplasmic Genetic Male Sterility:
When pollen sterility is controlled by both cytoplasmic and nuclear genes is known as cytoplasmic and nuclear genes is known as cytoplasmic genetic male sterility. Jones and Davis first discovered this type of male sterility in 1944 in onion.
This is the case of cytoplasmic male sterility, where a nuclear genes restoring fertility in the male sterile line is known. The fertility restore gene ‘R’ is dominant and found in certain strains of the species. This genes restores male fertility in the male sterile line, hence is known as restores gene.
This system includes A, B, and R lines. A line is a male sterile line, B is similar to ‘A’ in all features but it is a male fertile and R is restore line it restore the fertility in the F1 hybrid. since B line is used to maintain the fertility and is also referred as maintainer line. The plants would be male sterile line in the presence of male sterile cytoplasm if the nuclear genotype is rr, but would be male fertile if the nucleus is Rr or RR. New male sterile lines may be developed following the same procedure as in the case of cytoplasmic system, but the nuclear genotype of the pollinator strain used in transfer must be the fertility would be restored. Development of new restorer strain is somewhat indirect. First a restorer strain (R) is crossed with male sterile line. The resulting male fertile plants are used as the female parent in repeated backcrosses with the strain (C) used as the recurrent parent to which transfer of restorer gene is desired. In each generation, male sterile plants are discarded and the male fertile plants are used as female for back crosses. This acts as selection device for the restores gene R during the backcross programme. At the end of back cross programme a restorer line isogenic to the strain ‘C’ would be recovered.
Utilization in Plant Breeding:
Cytoplasmic genetic male sterility is widely used for hybrid seed production of both seed propagated species and vegetatively propagated species. It is used commercially to produce hybrid seed in maize, Bajara, cotton, rice, sunflower, jowar, etc.
SYNTHETIC SEEDS
Synthetic seeds are defined as artificially encapsulated somatic embryos, shoot buds, cell aggregates, or any other tissue that can be used for sowing as a seed and that possess the ability to convert into a plant under in vitro or ex vitro conditions and that retain this potential also after storage. In simple words synthetic seed contains an embryo produced by somatic embryogenesis enclosed within an artificial medium that supplies nutrients and is encased in an artificial seed covering.
The technology designed to combine the advantages of clonal propagation with those of seed propagation and storage. Also be as channel for new plant lines produced through biotechnology advances.
The first synthetic seeds were produced by Kitto and Janick in 1982 using carrot somatic embryos
In some of the horticultural crops seeds propagation is not successful due to;
- Heterozygosity of seeds particularly in cross pollinated crops
- Minute seed size eg; orchids
- Presence of reduced endosperm
- Some seeds require mycorrhizal fungi association for germination eg: orchids
- No seeds are formed
These crop species can be propagated by vegetative means like micro propagation and clonal propagation.
Characteristics of synthetic seeds
- High volume. Large scale propagation method
- Maintains genetic uniformity of plants
- Direct delivery of propagules to the field, thus eliminating transplants
- Lower cost per plant let
- Rapid multiplication of plants
Procedure for synthetic seed production:
The somatic embryos foe synthetic seeds are produced in the lab through culturing of somatic cells and treating with different hormones to produce root and shoot. The following are the different steps involved in artificial seeds production;
1) Establish somatic embryogenesis
2) Mature somatic embryos
3) Synchronize and singulate somatic embryos
4) Mass production of embryos
5) Encapsulation of matured somatic embryos
6) Desiccation
7) Field planting
Application of synthetic seeds
By combining the benefits of a vegetative propagation system with the capability of long-term storage and with the clonal multiplication, synthetic seeds have many diverse applications in the field.
1) Multiplication of non-seed producing plants, ornamental hybrids or polyploids plants
2) Propagation of male or female sterile plants for hybrid seed production
3) Germplasm conservation of recalcitrant species
4) Multiplication of transgenic
Limitations:
1) Limited production of viable micropropagules that are useful in synthetic seed production
2) Asynchrous development of somatic embryos
3) Improper maturation of somatic embryos that makes them inefficient for germination and conversion in to normal plants
4) Lack of dormancy and stress tolerance in somatic embryos that limit the storage of synthetic seeds
5) Somo clonal variations which may alter the genetic constituent of the embryos
Vector (molecular biology)
In molecular cloning, a vector is a DNA molecule used as a vehicle to artificially carry foreign genetic material into another cell, where it can be replicated and/or expressed. A vector containing foreign DNA is termed recombinant DNA. The four major types of vectors are plasmids, viral vectors, cosmids, and artificial chromosomes. Common to all engineered vectors are an origin of replication, a multicloning site, and a selectable marker.
The vector itself is generally a DNA sequence that consists of an insert (transgene) and a larger sequence that serves as the "backbone" of the vector. The purpose of a vector which transfers genetic information to another cell is typically to isolate, multiply, or express the insert in the target cell. Vectors called expression vectors (expression constructs) specifically are for the expression of the transgene in the target cell, and generally have a promoter sequence that drives expression of the transgene. Simpler vectors called transcription vectors are only capable of being transcribed but not translated: they can be replicated in a target cell but not expressed, unlike expression vectors. Transcription vectors are used to amplify their insert.
Insertion of a vector into the target cell is usually called transformation for bacterial cells, transfection for eukaryotic cells, although insertion of a viral vector is often called transduction.
Methods of gene transfer
A variety of gene transfer strategies have been developed during the last decade for the treatment of human diseases which can be grouped into the two major categories: the viral and non-viral methods.
A variety of gene transfer strategies have been developed during the last decade for the treatment of human diseases which can be grouped into the two major categories: the viral and non-viral methods.
(i) Virus vectors. After 1980, much work has been done on retroviruses as gene transfer vectors, more specifically on murine-leukemia virus (MLV) for gene therapy. Efforts are being made to develop HIV-based vectors so that even non-dividing cells can be injected. The outline of retroviral gene transfer has been shown in Fig. 5.8. The steps of developing a replication-defective recombinant retroviral vectors are : (i) the replacement of viral structural genes e.g. gag, pol and env by the therapeutic foreign genes of interest, (ii) transfection of this vector into packaging cell line (i.e. producer cells) that provide the viral structural proteins in trans so that the recombinant retroviral genome is packed and replication defective retroviruses are generated, (iii) transfection of host cells by such viruses, and reverse transcription of recombinant retroviral RNA and random integration into the host genome. In the absence of viral genes, the foreign genes (therapeutic in nature is transcribed from the viral LTRs, the long terminal repeats) and desired protein is synthesized (Fig. 5.8). The retroviral vectors are used in ex vivo gene transfer experiment, although it has been shown that they can infect a regenerating liver when administered intravenously into hepatectomized animal.
(ii) Non-viral approaches. Most of approaches in gene therapy have been focused on development of viral vectors rather than clinical efficacy and biosafety. Furthermore, techniques of virus-based gene therapy is very costly and complex. Therefore, this led to the development of non-viral gene therapy. Majority of non-viral methods follow the in vivo approaches. Therefore a gene can be used successfully as a drug. In general, the non-viral methods for gene transfer can be grouped into two methods, physical and chemical methods.
(a) Physical methods. The various forms of physical methods are given below :
Microinjection : It is a very tedious method. However, it is used in oocytes, eggs, and embryos. Jeffrey S. Chamberlain et al. (1993) of Human Genome Center, Michigan University U.S.A. have cured mice that inherited a neuro-muscular disease which is similar to muscular dystrophy of humans (for detail see 3.2.2.4). The mutant mice lacking 427 K gene in brain and muscle cells had a weak diaphragm which gradually degenerates like that in dystrophy affected humans. Chamberlain and coworkers microinjected the DNA containing 427 K gene into the zygote of mutant mice. The transplanted genes worked properly and produced necessary protein and thus, prevented the diaphragm.
Microinjection : It is a very tedious method. However, it is used in oocytes, eggs, and embryos. Jeffrey S. Chamberlain et al. (1993) of Human Genome Center, Michigan University U.S.A. have cured mice that inherited a neuro-muscular disease which is similar to muscular dystrophy of humans (for detail see 3.2.2.4). The mutant mice lacking 427 K gene in brain and muscle cells had a weak diaphragm which gradually degenerates like that in dystrophy affected humans. Chamberlain and coworkers microinjected the DNA containing 427 K gene into the zygote of mutant mice. The transplanted genes worked properly and produced necessary protein and thus, prevented the diaphragm.
Direct DNA injection. Direct injection of DNA into skeletal muscle has created a lot of excitement and led to the possibility of using gene as vaccines. Direct gene transfer was attempted since early 1960s but these studies were not done seriously due to inefficient gene transfer and low level of expression. Due to low level of expression therapeutic benefits for the treatment of genetic disorder could not be derived.
Thereafter, these studies were further carried out. The low levels of foreign proteins synthesized in the host cells were processed along the class I MHC pathway leading to presentation of peptides on cell surface resulting in a potent immune response against the foreign antigens. This gave the birth to the concept of DNA vaccine or genetic immunization (Rangarajan and Padmanaban, 1996) (see DNA Vaccines).
Thereafter, these studies were further carried out. The low levels of foreign proteins synthesized in the host cells were processed along the class I MHC pathway leading to presentation of peptides on cell surface resulting in a potent immune response against the foreign antigens. This gave the birth to the concept of DNA vaccine or genetic immunization (Rangarajan and Padmanaban, 1996) (see DNA Vaccines).
Gene gun. It was developed originally for transformation of plant cells, but in animal system it is also being used for delivery of genes into tissues such as liver, skin, mammary glands and muscle. Direct gene delivery is now being used to map promoter elements that regulate gene expression in vivo, in transfection of herpes simplex thymidine kinase genes into melanomas to render them sensitive to gancyclovir and as a vaccination strategy. Thus, direct gene transfer technique failed to make an impression in treatment of genetic disorders but made enormous impact in the field of vaccine development. The other physical methods e.g. microinjection, electroporation, etc. have the limited scope in humans.
Magic bullets - cells as drug delivery system. Magic bullet is a drug delivery system through a cell loaded with an appropriate treatment molecule which upon injection into a patients migrates through the circulatory system directly to the diseased site. Still, it is only a dream to the patients suffering from genetic diseases. In future it may be possible to cure such diseases such as cystic fibrosis, when a cell can reach to the lung tissues and deliver an enzymes to breakdown the thick getatinous mucus, and help in breath and food digestion.
(b) Chemical methods. Some times chemicals are used to make easy delivery of DNA into recipient cell.
Using detergent mixture. Certain charged chemical compounds e.g. calcium phosphate, dextran or lipids are mixed with functional cDNA of desired function. The mixture is introduced near the vicinity of recipient cells. Thereafter, it is spread to the interior of the body organ. The chemical mixed with cDNA disturbs the cell membranes, widens pore size and allows the cDNA to pass into the cell. In this method the number of cells allowing the entry of cDNA is very small.
Using detergent mixture. Certain charged chemical compounds e.g. calcium phosphate, dextran or lipids are mixed with functional cDNA of desired function. The mixture is introduced near the vicinity of recipient cells. Thereafter, it is spread to the interior of the body organ. The chemical mixed with cDNA disturbs the cell membranes, widens pore size and allows the cDNA to pass into the cell. In this method the number of cells allowing the entry of cDNA is very small.
Receptor-meditated gene delivery. It is an attractive strategy. It takes advantage of normal physiological pathway. The receptors are exclusively present on hepatocytes. They bind to certain glvcoproteins lacking the terminal asialic acid. The concept of delivering genes into specific tissue was examined extensively with the liver asialoglycoprotein receptors. An asialoglycoprotein (orosomucoid) is coupled with poly-L-lysine. This conjugate is then condensed with plasmid DNA via electrostatic interactions (Fig. 5.10). This soluble complex i.e. asialoglycoprotein-poly-L-lysin-DNA complex (ASGP-PL-DNA) is injected intravenously, intraperitoneally, which gets entered into hepatocytes via the asialoglycoprotein receptors (ASGPR). DNA is delivered into the nucleus. The transgene expression occurs, therefore, recombination protein is detected for several weeks (Wu and Wu, 1988).
Embryo therapy through IVF-technology. In 1993, adopting IVF-technology, Dr. A. Handyside (a team leader of British Researcher at Hammersmith Hospital, London) got success in producing a genetically engineered female baby, whose parents transmitted the genetic disease (cystic fibrosis) in earlier four babies, who died later on. Even after confirmation through prenatal diagnosis that the babies would be sufferer or carrier of this disease they decided to give birth to babies, which failed. This disease clogs the lungs of sufferer and makes them unable to digest the food properly.
Micropropagation
Micropropagation is the practice of rapidly multiplying stock plant material to produce a large number of progeny plants, using modern plant tissue culture methods.[1]
Micropropagation is used to multiply novel plants, such as those that have been genetically modified or bred through conventional plant breeding methods. It is also used to provide a sufficient number of plantlets for planting from a stock plant which does not produce seeds, or does not respond well to vegetative reproduction.
Method (Stages of micropropagation)
[edit] Establishment
Micropropagation begins with the selection of plant material to be propagated. Clean stock materials that are free of viruses and fungi are important in the production of the healthiest plants. Once the plant material is chosen for culture, the collection of explant(s) begins and is dependent on the type of tissue to be used; including stem tips, anthers, petals, pollen and others plant tissues. The explant material is then surface sterilized, usually in multiple courses of bleach and alcohol washes, and finally rinsed in sterilized water. This small portion of plant tissue, sometimes only a single cell, is placed on a growth medium, typically containing sucrose as an energy source and one or more plant growth regulators (plant hormones). Usually the medium is thickened with agar to create a gel which supports the explant during growth. Some plants are easily grown on simple media, but others require more complicated media for successful growth; the plant tissue grows and differentiates into new tissues depending on the medium. For example, media containing cytokinins are used to create branched shoots from plant buds.
[edit] Multiplication
Multiplication is the taking of tissue samples produced during the first stage and increasing their number. Following the successful introduction and growth of plant tissue, the establishment stage is followed by multiplication. Through repeated cycles of this process, a single explant sample may be increased from one to hundreds or thousands of plants. Depending on the type of tissue grown, multiplication can involve different methods and media. If the plant material grown is callus tissue, it can be placed in a blender and cut into smaller pieces and recultured on the same type of culture medium to grow more callus tissue. If the tissue is grown as small plants called plantlets, hormones are often added that cause the plantlets to produce many small offshoots that can be removed and recultured.
[edit] Pretransplant
Banana plantlets transferred to soil (with vermicompost) from plant media. This process is done for acclimatization of plantlets to the soil as they were previously grown in plant media. After growing for some days the plantlets are transferred to the field.
This stage involves treating the plantlets/shoots produced to encourage root growth and "hardening." It is performed in vitro, or in a sterile "test tube" environment.
"Hardening" refers to the preparation of the plants for a natural growth environment. Until this stage, the plantlets have been grown in "ideal" conditions, designed to encourage rapid growth. Due to the controlled nature of their maturation, the plantlets often do not have fully functional dermal coverings. This causes them to be highly susceptible to disease and inefficient in their use of water and energy. In vitro conditions are high in humidity, and plants grown under these conditions often do not form a working cuticle and stomata that keep the plant from drying out. When taken out of culture, the plantlets need time to adjust to more natural environmental conditions. Hardening typically involves slowly weaning the plantlets from a high-humidity, low light, warm environment to what would be considered a normal growth environment for the species in question.
[edit] Transfer from culture
In the final stage of plant micropropagation, the plantlets are removed from the plant media and transferred to soil or (more commonly) potting compost for continued growth by conventional methods.
This stage is often combined with the "pretransplant" stage.
[edit] Advantages
Micropropagation has a number of advantages over traditional plant propagation techniques:
- The main advantage of micropropagation is the production of many plants that are clones of each other.
- Micropropagation can be used to produce disease-free plants.
- Micropropagation produces rooted plantlets ready for growth, saving time for the grower when seeds or cuttings are slow to establish or grow.
- It can have an extraordinarily high fecundity rate, producing thousands of propagules while conventional techniques might only produce a fraction of this a number.
- It is the only viable method of regenerating genetically modified cells or cells after protoplast fusion.
- It is useful in multiplying plants which produce seeds in uneconomical amounts, or when plants are sterile and do not produce viable seeds or when seed cannot be stored (see recalcitrant seeds).
- Micropropagation often produces more robust plants, leading to accelerated growth compared to similar plants produced by conventional methods - like seeds or cuttings.
- Some plants with very small seeds, including most orchids, are most reliably grown from seed in sterile culture.
- A greater number of plants can be produced per square meter and the propagules can be stored longer and in a smaller area.
[edit] Disadvantages
Micropropagation is not always the perfect means of multiplying plants. Conditions that limits its use include:
- It is very expensive, and can have a labor cost of more than 70%
- A monoculture is produced after micropropagation, leading to a lack of overall disease resilience, as all progeny plants may be vulnerable to the same infections.
- An infected plant sample can produce infected progeny. This is uncommon as the stock plants are carefully screened and vetted to prevent culturing plants infected with virus or fungus.
- Not all plants can be successfully tissue cultured, often because the proper medium for growth is not known or the plants produce secondary metabolic chemicals that stunt or kill the explant.
- Sometimes plants or cultivars do not come true to type after being tissue cultured. This is often dependent on the type of explant material utilized during the initiation phase or the result of the age of the cell or propagule line.
- Some plants are very difficult to disinfect of fungal organisms.
The major limitation in the use of micropropagation for many plants is the cost of production; for many plants the use of seeds, which are normally disease free and produced in good numbers, readily produce plants (see orthodox seed) in good numbers at a lower cost. For this reason, many plant breeders do not utilize micropropagation because the cost is prohibitive. Other breeders use it to produce stock plants that are then used for seed multiplication.
Mechanisation of the process could reduce labour costs, but has proven difficult to achieve, despite active attempts to develop technological solutions.
Ovule Culture
Ovule culture is an elegant experimental system by which ovules are aseptically isolated from the ovary and are grown aseptically on chemically defined nutrient medium under controlled conditions.
Principle:
An ovule is a megasporangium covered by integument. An ovule contains a megaspore or an egg cell. After fertilization a single cell zygote is formed which ultimately leads to form a mature embryo possessing shoots and root primordia.
In Vitro ovule culture helps to understand the factors that regulate the development of zygote through organised stages to a mature embryo. Alternatively, it may be possible to germinate pollen in the same culture as the excised and to induce in vitro fertilization and subsequent embryo production.
Importance of Ovule Culture
1. Test Tube Pollination and Fertilization:
Through ovule culture, test tube pollination and fertilization can be done. By technique, it may be possible to germinate pollen in the same culture as the excised ovule and to induce a vitro fertilization leading to the formation of mature seeds containing viable embryos.
2. Application in Hybridization:
Ovule culture has been successfully employed to obtain hybrid seedlings in Interspecific and Intergeneric crosses. In several interspecific crosses, the hybrid embryo of Abelmoschus fails to develop beyond the heart or torpedo-shaped embryo. By ovule cultures, viable hybrids have been obtained in three out of five crosses attempted in Abelmoschus species.
Although hybrid plants have not been obtained between different species of cotton through fertilized ovule culture, but seed development and the production of fibre from the cultures ovule have been demonstrated.
3. Production of Haploid Callus:
It is possible to obtain haploid callus by culturing unfertilized ovules.
4. Ovule Cultures of Orchid Plants:
In nature, the seeds of orchid germinate only in association with a proper fungus. As a result numerous seeds are lost due to unavailability of proper fungus. Besides this the seed capsule of many orchids takes a long time to mature. To overcome such problems, several attempts have been made to culture the fertilized ovule of orchid in vitro.
5. Induction of Polyembryos:
In horticultural practises, the artificial induction of polyembryos holds a great potential. It has been observed that the nucellus of mono-embryonic ovules of citrus can be induced to form adventive embryos in culture.
6. Virus Irradication:
In the varieties of citrus which are impossible to free of virus by other means, the ovule culture has proved decisively advantages to make them virus free.
Ti plasmid
The structure of the Ti plasmid
A Ti or tumour inducing plasmid is a circular plasmid that often, but not always, is a part of the genetic equipment that Agrobacterium tumefaciens and Agrobacterium rhizogenes use to transduce its genetic material to plants. The Ti plasmid is lost when Agrobacterium is grown above 28°C. Such cured bacteria do not induce crown galls, i.e. they become avirulent. pTi and pRi share little sequence homology but are functionally rather similar. The Ti plasmids are classified into different types based on the type of opine produced by their genes. The different opines specified by pTi are octopine, nopaline, succinamopine and leucinopine.
The plasmid has 196 genes that code for 195 proteins. There is no one structural RNA. The plasmid is 206,479 nucleotides long, the GC content is 56% and 81% of the material is coding genes. There are no pseudogenes.
The modification of this plasmid is very important in the creation of transgenic plants, but only in dicotyledon plants.
Virulence Region
Genes in the virulence region are grouped into the operons virABCDEFG, which code for the enzymes responsible for mediating transduction of T-DNA to plant cells.[1]
- virA codes for a receptor which reacts to the presence of phenolic compounds such as acetosyringone,[2] syringealdehyde or acetovanillone[3] which leak out of damaged plant tissues.[4]
- virD1 and virD2 produce endonucleases which target the direct repeat borders of the T-DNA segment,
[edit] Characteristics Features
- Agrobacterium is called the natural genetic engineer.
- Size of the plasmid: ~250 kbp.
- Contains a region enabling conjugative transfer.
- Contains regions for opine synthesis and catabolism.
- Responsible for crown gall disease in plants.
Ri Plasmids
Agrobacterium rhizogenes is a
Gram negative soil bacterium that produces hairy root disease in dicotyledonous plants. A. rhizogenes induces the formation of proliferative multi-branched adventitious roots at the site of infection; so called 'hairy roots' [2]
Gram negative soil bacterium that produces hairy root disease in dicotyledonous plants. A. rhizogenes induces the formation of proliferative multi-branched adventitious roots at the site of infection; so called 'hairy roots' [2]
In the rhizosphere, plants may suffer from wounds by soil pathogens or other sources. This leads to the secretion of phenolic compounds like acetosyringone which have chemotactic effects that attract the bacteria. Under such conditions, certain bacterial genes are turned on leading to the transfer of its T-DNA from its root inducing plasmid (Ri plasmid) into the plant through the wound. After integration and expression, in vitro or under natural conditions, the hairy root phenotype is observed, which typically includes overdevelopment of a root system that is not completely geotropic, and altered (wrinkled) leaf morphology, if leaves are present.[3]
The
hairy roots are grown in vitro in bioreactors to study their soil interaction with other pathogens like fungi and nematodes. This technique has also led to the commercial production of certain metabolic compounds that the plant is known to secrete, especially in regard to the medicinal plants that are difficult to cultivate in sufficient quantities by other means.[5] The root cultures are also used for
genetic engineering.
hairy roots are grown in vitro in bioreactors to study their soil interaction with other pathogens like fungi and nematodes. This technique has also led to the commercial production of certain metabolic compounds that the plant is known to secrete, especially in regard to the medicinal plants that are difficult to cultivate in sufficient quantities by other means.[5] The root cultures are also used for
genetic engineering.
Principles of Green House Technology:
A greenhouse (also called a glasshouse) is a building where plants are grown under controlled micro environment. These structures range in size from small sheds to very large buildings. A miniature greenhouse is known as a cold frame.
A greenhouse is a structure with different types of covering materials, such as a glass or plastic roof and frequently glass or plastic walls; it heats up because incoming visible solar radiation (for which the glass is transparent) from the sun is absorbed by plants, soil, and other things inside the building. Air warmed by the heat from hot interior surfaces is retained in the building by the roof and wall.
Purpose of the Green House
a) To promote tomato growing in the cooler areas
b) To promote the growing of tomatoes throughout the year in both cool and warm areas
Advantages of a Green House
a) Higher yields can be realized/intensive production per unit area
b) High quality produce
c) Minimized cases of diseases
d) Market timing for optimum profit
e) Production levels may be maintained all the year round
f) Other warm season crops may be grown throughout the year
Other Crops Recommended in the Green House
a) Coloured Capsicum varieties e.g. red, yellow etc.
b) Cucumbit family e.g. Cucumbers, courgettes
c) Onions e.g. bulb onions,
d) Garlics
e) Herbs and Spices e.g coriander, parsley, celery
f) Brinjals (egg plants)
Green House Methodology:
Types of Green Houses
Classification According to Size
a) Cold frame (miniature) green house for subsistence (less than 15×7 m)
b) Economic unit - 15mx7m
c) Commercia l- more than 15×7m
Classification according to lifespan and materials used
Frames can be covered with glass, rigid fiberglass, rigid double-wall plastics, or plastic film
a) Permanent green houses
Metal frame-work and covered with glass with automated cooling/heating systems e.g. in research stations
b) Semi permanent green houses
Metal frame-work but covered with plastic film/paper
Recommended for established farmers
c) Temporary green houses
Wooden frame-work covered with plastic film /paper.
This is recommended for small scale farmers.
Classification according to structural shape of frame
A greenhouse can be attached to a house or garage; it can be a freestanding structure or gutter connected
a) Attached Greenhouses
i) Lean-to. A lean-to greenhouse is a half greenhouse, split along the peak of the roof, or ridge line (Figure 2A), Lean-tos are useful where space is limited to a width of approximately seven to twelve feet, and they are the least expensive structures.
Finally, consider the location of windows and doors on the supporting structure and remember that heavy rain might slide off the roof of the house onto the structure.
ii) Even-span. An even-span is a full-size structure that has one gable end attached to another building (Figure 2B). It is usually the largest and most costly option, but it provides more usable space and can be lengthened. The even-span has a better shape than a lean-to for air circulation to maintain uniform temperatures. An even-span can accommodate two to three benches for growing crops.
iii) Window-mounted. A window-mounted greenhouse can be attached on the south or east side of a house. This glass enclosure gives space for conveniently growing a few plants at relatively low cost (Figure 2D). The special window extends outward from the house a foot or so and can contain two or three shelves.
b) Freestanding (detached) Structures:
Freestanding greenhouses are separate structures; they can be set apart from other buildings to get more sun and can be made as large or small as desired.
Somaclonal variation
Somaclonal variation is the variation seen in plants that have been produced by plant tissue culture. Chromosomal rearrangements are an important source of this variation.
Somaclonal variation is not restricted to, but is particularly common in, plants regenerated from callus. The variations can be genotypic or phenotypic, which in the latter case can be either genetic or epigenetic in origin. Typical genetic alterations are: changes in chromosome numbers (polyploidy and aneuploidy), chromosome structure (translocations, deletions, insertions and duplications) and DNA sequence (base mutations). Typical epigenetic related events are: gene amplification and gene methylation.
If no visual, morphogenic changes are apparent, other plant screening procedures must be applied. There are both benefits and disadvantages to somaclonal variation. The phenomenon of high variability in individuals from plant cell cultures or adventitious shoots has been named somaclonal variation.
Factors affecting somaclonal variations:
1) method of vegetative propagation
2) tissue culture medium
3) explant material
4) growth regulators
5) number of passages
6) environmental factors like light, temp. etc.
7) genetic constitution.
Application of Somaclonal Variation
1. Somaclonal variation and gametoclonal variation represent useful source of introducing genetic variations that could be of value to plant breeders.
2. Single gene mutation in nuclear or organelle genome may give the best available variety in vitro that has a specific character.
3. Gametoclonal variation, induced mostly by meiotic recombination during the sexual cycle of F1 hybrid, results in trasngressive segregation to uncover unique gene combination.
4. Various cell lines selected in vitro may prove potentially applicable to agriculture and industry like resistance to herbicide, pathotoxin, salt or aluminium.
5. Variability in cell cultures has played a useful role in synthesis of secondary metabolites on a commercial scale.
5. Variability in cell cultures has played a useful role in synthesis of secondary metabolites on a commercial scale.
6. Technique employed for Somaclonal and gametoclonal variation are relatively easier than recombinant DNA technique.
Somaclonal variants for agronomically desirable traits in several crop plants have been raised from tissue culture. Some examples of Somaclonal variation in crop plants as well as in some horticulturally important plants are given below:
Rice:
Significant improvements relative to parent were observed for seed weight, seed proteins percentage, tiller number, panicle length and time of flowering. At IRRI, mutants were observed for many characters such as panicle, grain, and leaf morphology and tiller arrangement.
Wheat:
Variations were manifested for gliadin proteins in seed, grain colour, plant height, heading date and yield.
Maize:
Plants regenerated from selected cell lines were resistant both to T-toxin and to infection to Drechslera maydis causing southern leaf blight. Cytoplasmic male sterile lines are very sensitive to the T-toxin produced by Drechslera maydis.
Potato:
Somaclonal variants were selected for resistance to Phytopthora infestans and to its multiple races and resistance to early blight.
Tomato:
Somaclones were isolated with variant phenotypes, such as recessive mutation for male sterility, resistance to Fusarium oxysporium, jointless pedicel , tangerine virescent leaf, flower and fruit colour.
Microinjection
Microinjection refers to the process of using a glass micropipette to insert substances at a microscopic or borderline macroscopic level into a single living cell. It is a simple mechanical process in which a needle roughly 0.5 to 5 micrometers in diameter penetrates the cell membrane and/or the nuclear envelope. The desired contents are then injected into the desired sub-cellular compartment and the needle is removed. Microinjection is normally performed under a specialized optical microscope setup called a micromanipulator. The process is frequently used as a vector in genetic engineering and transgenics to insert genetic material into a single cell. Microinjection can also be used in the cloning of organisms, and in the study of cell biology and viruses.
[edit] Subtypes
[edit] Pronuclear Injection
Pronuclear injection is a technique used to create transgenic organisms by injecting genetic material into nucleus of a fertilized oocyte. This technique is commonly used to study the role of genes using mouse models.
[edit] Pronuclear Injection in Mice
In order for pronuclear injection to be successful, the genetic material (typically linear DNA) must be injected while the genetic material from the oocyte and sperm are separate (i.e., the pronuclear phase).[1] In order to obtain these oocytes, mice are commonly superovulated using gonadotrophins.[2] Once plugging has occurred, oocytes are harvested from the mouse and injected with the genetic material. The oocyte is then implanted in the oviduct of a pseudopregnant animal.[1] While efficiency varies, 10-40% of mice born from these implanted oocytes may contain the injected construct.[2] Transgenic mice can then be bred to create transgenic lines.
[edit] Problems with Pronuclear Injection
The incorporation of injected DNA into the mouse genome cannot be tightly controlled. As a result, improper incorporation may occur. If DNA is incorporated at the 2-cell stage, some cells may express the DNA while others will not (so called mosaic mice).[2] In addition, if the injected DNA is incorporated in multiple sites, multiple copies of the DNA may be expressed leading to overexpression.[2] Even if only one copy of DNA is incorporated into the genome, if it is not incorporated into the germline, then it will not be passed to offspring.[2]
[edit] Protoplast Injection
Electroporation
Electroporation, or electropermeabilization, is a significant increase in the electrical conductivity and permeability of the cell plasma membrane caused by an externally applied electrical field. It is usually used in molecular biology as a way of introducing some substance into a cell, such as loading it with a molecular probe, a drug that can change the cell's function, or a piece of coding DNA.[1]
Electroporation is a dynamic phenomenon that depends on the local transmembrane voltage at each point on the cell membrane. It is generally accepted that for a given pulse duration and shape, a specific transmembrane voltage threshold exists for the manifestation of the electroporation phenomenon (from 0.5 V to 1 V). This leads to the definition of an electric field magnitude threshold for electroporation (Eth). That is, only the cells within areas where E≧Eth are electroporated. If a second threshold (Eir) is reached or surpassed, electroporation will compromise the viability of the cells, i.e., irreversible electroporation.[2]
In molecular biology, the process of electroporation is often used for the transformation of bacteria, yeast, and plant protoplasts. In addition to the lipid membranes, bacteria also have cell walls which are different from the lipid membranes and are made of peptidoglycan and its derivatives. However, the walls are naturally porous and only act as stiff shells that protect bacteria from severe environmental impacts. If bacteria and plasmids are mixed together, the plasmids can be transferred into the cell after electroporation. Several hundred volts across a distance of several millimeters are typically used in this process. Afterwards, the cells have to be handled carefully until they have had a chance to divide producing new cells that contain reproduced plasmids. This process is approximately ten times as effective as chemical transformation.[1][3]
This procedure is also highly efficient for the introduction of foreign genes in tissue culture cells, especially mammalian cells. For example, it is used in the process of producing knockout mice, as well as in tumor treatment, gene therapy, and cell-based therapy. The process of introducing foreign DNAs into eukaryotic cells is known as transfection.
Advantages:
Versatility: Electroporation is effective with nearly all cell and species types (Nickoloff, 1995).
Efficiency: A large majority of cells take in the target DNA or molecule. In a study on electrotransformation of E. coli, for example, 80% of the cells received the foreign DNA (Miller and Nickoloff, 1995).
Small Scale: The amount of DNA required is smaller than for other methods (Withers, 1995)
In vivo: The procedure may be performed with intact tissue (Weaver, 1995). A paper published in Developmental Biology showed the successful transfer of a DNA construct with a fluorescent reporter gene into intact mouse brain tissue (Fig 4) (Saito, 2001).
Applications
As previously mentioned, electroporation is widely used in many areas of molecular biology research and in the medical field. Some applications of electroporation include:
- DNA Transfection or Transformation: This is likely the most widespread use of electroporation. Specific genes can be cloned into a plasmid and then this plasmid introduced into host cells (bacterial or otherwise) in order to investigate gene and protein structure and function. (Nickoloff, 1995)
- Direct Transfer of Plasmids Between Cells: Bacterial cells already containing a plasmid may be incubated with another strain that does not contain plasmids but that has some other desireable feature.
- Induced Cell Fusion: The disruption of the membrane that occurs with the quick pulse of electricity in the electroporation procedure has also been shown to induce fusion of cells (Weber and Berg, 1995).
- Trans-dermal Drug Delivery: Just as electroporation causes temporary pores to form in plasma membranes, studies suggest that similar pores form in lipid bilayers of the stratum corneum- the outermost dead layer of skin. These pores could allow drugs to pass through to the skin to a target tissue. This method of drug delivery would be more pleasant than injection for the patient (not requiring a needle) and could avoid the problems of improper absorption or degradation of oral medication in the digestive system (Praustnitz et. al., 1993).
- Cancer Tumor Electrochemotherapy: Scientists are investigating the potential of electroporation to increase the effectiveness of chemotherapy.
- Gene Therapy: Much like drug delivery, electroporation techniques can allow vectors containing important genes to be transported across the skin and into the target tissue. Once incorporated into the cells of the body, the protein produced from this gene could replace a defective one and thus treat a genetic disorder.
Hairy Roots
Hairy root - a phase of crown gall (especially in apples) during which there is abnormal development of fine fibrous roots
crown gall - a bacterial disease of plants which forms excrescences on the stem near the ground
Hairy root culture, also called transformed root culture, is a type of plant tissue culture that is used to study plant metabolic processes or to produce valuable secondary metabolites, often with plant genetic engineering.
A naturally occurring soil bacterium Agrobacterium rhizogenes that contains root inducing plasmids (also called Ri plasmids) can infect plant roots and cause them to produce a food source for the bacterium, opines, and to grow abnormally. The abnormal roots are particularly easy to culture in artificial media because hormones are not needed, and they are neoplastic, with indefinite growth. The neoplastic roots produced by A. rhizogenes infection have a high growth rate (compared to untransformed adventitious roots), as well as genetic and biochemical stability.
Production of hairy roots in vivo:
- Agrobacterium recognizes some signal molecules exuded by wounded plant cells and becomes attached to it.
- The bacteria contain the Root inducing plasmid (Ri-plasmid)
- The bacteria genetically transfer part of the Ri-plasmid called the transfer DNA (T-DNA) to the plant genome, where it is get expressed and make the plant cell to:
- Proliferate by increasing the rate of cell division (cytokinie expession) and cell elongation (auxin expression) to produce the hairy roots.
- Produce the opines which is a type of unusual amino acids (octopine, agropine,nopaline, mannopine, and cucumopine) which is used by the bacterium as a carbon, nitrogen and energy source
Structure of Ri plasmid
Induction of hairy root cultures in vitro:
- Explants are wounded and then inoculated with Agrobacterium rhizogenes.
- Usually two or three days later, the explant can be transferred into solid media with antibiotics, such as cefotaxime, vancomycin or ampicillin to kill or eliminate redundant bacteria.
- The hairy roots will be induced within a short period of time, which varies from one week to over a month depending on different plant species.
- The decontaminated hairy roots can be sub cultured on phytohormone-free medium.
Advantages of hairy root cultures:
- The hairy root system is genetically and biosynthetically stable
- High production of secondary metabolites
- The culture can grow under phyto-hormone-free conditions.
- The culture shows fast growth which reduce the culture time and easy the handling
Applications of hairy root cultures:
- Functional analysis of genes
- Expressing foreign proteins
- Production of secondary metabolites
- The culture may Produce compounds which is not found in untransformed roots
- The culture may change the composition of metabolites
- The culture can be used to regenerate a whole plants
Sterilization (microbiology)
Sterilization (or sterilisation) is a term referring to any process that eliminates (removes) or kills all forms of microbial life, including transmissible agents (such as fungi, bacteria, viruses, spore forms, etc.) present on a surface, contained in a fluid, in medication, or in a compound such as biological culture media.[1][2] Sterilization can be achieved by applying the proper combinations of heat, chemicals, irradiation, high pressure, and filtration.
[edit] Methods of sterilization
[edit] Heat sterilization
[edit] Steam sterilization utensils
A widely-used method for heat sterilization is the autoclave, sometimes called a converter. Autoclaves commonly use steam heated to 121–134 °C (250–273 °F). To achieve sterility, a holding time of at least 15 minutes at 121 °C (250 °F) or 3 minutes at 134 °C (273 °F) is required. Additional sterilizing time is usually required for liquids and instruments packed in layers of cloth, as they may take longer to reach the required temperature (unnecessary in machines that grind the contents prior to sterilization). Following sterilization, liquids in a pressurized autoclave must be cooled slowly to avoid boiling over when the pressure is released. Modern converters operate around this problem by gradually depressing the sterilization chamber and allowing liquids to evaporate under a negative pressure, while cooling the contents.
[edit] Heat sterilization of foods
Although imperfect, cooking and canning are the most common applications of heat sterilization. Boiling water kills the vegetative stage of all common microbes. Roasting meat until it is well done typically completely sterilizes the surface. Since the surface is also the part of food most likely to be contaminated by microbes, roasting usually prevents food poisoning. Note that the common methods of cooking food do not sterilize food - they simply reduce the number of disease-causing micro-organisms to a level that is not dangerous for people with normal digestive and immune systems.
[edit] Other heat sterilization methods
Flaming is done to loops and straight-wires in microbiology labs. Leaving the loop in the flame of a Bunsen burner or alcohol lamp until it glows red ensures that any infectious agent gets inactivated. This is commonly used for small metal or glass objects, but not for large objects (see Incineration below). However, during the initial heating infectious material may be "sprayed" from the wire surface before it is killed, contaminating nearby surfaces and objects. Therefore, special heaters have been developed that surround the inoculating loop with a heated cage, ensuring that such sprayed material does not further contaminate the area. Another problem is that gas flames may leave residues on the object, e.g. carbon, if the object is not heated enough.
Incineration will also burn any organism to ash. It is used to sanitize medical and other biohazardous waste before it is discarded with non-hazardous waste.
Boiling in water for fifteen minutes will kill most vegetative bacteria and inactivate viruses, but boiling is ineffective against prions and many bacterial and fungal spores; therefore boiling is unsuitable for sterilization. However, since boiling does kill most vegetative microbes and viruses, it is useful for reducing viable levels if no better method is available. Boiling is a simple process, and is an option available to most people, requiring only water, enough heat, and a container that can withstand the heat; however, boiling can be hazardous and cumbersome.
Tindalization[6] /Tyndallization[7] named after John Tyndall is a lengthy process designed to reduce the level of activity of sporulating bacteria that are left by a simple boiling water method. The process involves boiling for a period (typically 20 minutes) at atmospheric pressure, cooling, incubating for a day, boiling, cooling, incubating for a day, boiling, cooling, incubating for a day, and finally boiling again. The three incubation periods are to allow heat-resistant spores surviving the previous boiling period to germinate to form the heat-sensitive vegetative (growing) stage, which can be killed by the next boiling step. This is effective because many spores are stimulated to grow by the heat shock. The procedure only works for media that can support bacterial growth - it will not sterilize plain water. Tindalization/tyndallization is ineffective against prions.
Dry heat can be used to sterilize items, but as the heat takes much longer to be transferred to the organism, both the time and the temperature must usually be increased, unless forced ventilation of the hot air is used. The standard setting for a hot air oven is at least two hours at 160 °C (320 °F). A rapid method heats air to 190 °C (374 °F) for 6 minutes for unwrapped objects and 12 minutes for wrapped objects.[8][9] Dry heat has the advantage that it can be used on powders and other heat-stable items that are adversely affected by steam (for instance, it does not cause rusting of steel objects).
[edit] Chemical sterilization
Chemicals are also used for sterilization. Although heating provides the most reliable way to rid objects of all transmissible agents, it is not always appropriate, because it will damage heat-sensitive materials such as biological materials, fiber optics, electronics, and many plastics. Low temperature gas sterilizers function by exposing the articles to be sterilized to high concentrations (typically 5 - 10% v/v) of very reactive gases (alkylating agents such as ethylene oxide, and oxidizing agents such as hydrogen peroxide and ozone).
[edit] Ethylene oxide
Ethylene oxide (EO or EtO) gas is commonly used to sterilize objects sensitive to temperatures greater than 60 °C and / or radiation such as plastics, optics and electrics. Ethylene oxide treatment is generally carried out between 30 °C and 60 °C with relative humidity above 30% and a gas concentration between 200 and 800 mg/l, and typically lasts for at least three hours. Ethylene oxide penetrates well, moving through paper, cloth, and some plastic films and is highly effective. EtO can kill all known viruses, bacteria and fungi, including bacterial spores and is compatible with most materials (e.g. of medical devices), even when repeatedly applied. However, it is highly flammable, toxic and carcinogenic.
[edit] Ozone
Ozone is used in industrial settings to sterilize water and air, as well as a disinfectant for surfaces. It has the benefit of being able to oxidize most organic matter. On the other hand, it is a toxic and unstable gas that must be produced on-site, so it is not practical to use in many settings.
[edit] Bleach
Chlorine bleach is another accepted liquid sterilizing agent. Household bleach consists of 5.25% sodium hypochlorite. It is usually diluted to 1/10 immediately before use; however to kill Mycobacterium tuberculosis it should be diluted only 1/5, and 1/2.5 (1 part bleach and 1.5 parts water) to inactivate prions. The dilution factor must take into account the volume of any liquid waste that it is being used to sterilize.[21] Bleach will kill many organisms immediately, but for full sterilization it should be allowed to react for 20 minutes. Bleach will kill many, but not all spores. It is also highly corrosive.
[edit] Glutaraldehyde and formaldehyde
Glutaraldehyde and formaldehyde solutions (also used as fixatives) are accepted liquid sterilizing agents, provided that the immersion time is sufficiently long. To kill all spores in a clear liquid can take up to 22 hours with glutaraldehyde and even longer with formaldehyde. The presence of solid particles may lengthen the required period or render the treatment ineffective. Sterilization of blocks of tissue can take much longer, due to the time required for the fixative to penetrate. Glutaraldehyde and formaldehyde are volatile, and toxic by both skin contact and inhalation. Glutaraldehyde has a short shelf life (<2 weeks), and is expensive. Formaldehyde is less expensive and has a much longer shelf life if some methanol is added to inhibit polymerization to paraformaldehyde, but is much more volatile. Formaldehyde is also used as a gaseous sterilizing agent; in this case, it is prepared on-site by depolymerization of solid paraformaldehyde. Many vaccines, such as the original Salk polio vaccine, are sterilized with formaldehyde.
[edit] Phthalaldehyde
Ortho-phthalaldehyde (OPA) is a chemical sterilizing agent that received Food and Drug Administration (FDA) clearance in late 1999. Typically used in a 0.55% solution, OPA shows better myco-bactericidal activity than glutaraldehyde. It also is effective against glutaraldehyde-resistant spores. OPA has superior stability, is less volatile, and does not irritate skin or eyes, and it acts more quickly than glutaraldehyde. On the other hand, it is more expensive, and will stain proteins (including skin) gray in color. Some side effects from equipment sterilized using this reagent have been reported. For example, two cases of anaphylaxis following cystoscopy with endoscopes sterilized with OPA were reported by Cooper, et al., (J Endourol. 2008 Sep;22(9):2181-4), and four cases of ortho-phthalaldehyde-induced anaphylaxis after laryngoscopy with the detection of specific IgE in serum were reported by Suzukawa, et al., (Allergol Int. 2007 Sep;56(3):313-6. Epub 2007 Jul 1; J Allergy Clin Immunol. 2006 Jun;117(6):1500-1. Epub 2006 Mar 31).
[edit] Hydrogen peroxide
Hydrogen peroxide is another chemical sterilizing agent. It is relatively non-toxic when diluted to low concentrations, such as the familiar 3% retail solutions although hydrogen peroxide is a dangerous oxidizer at high concentrations (> 10% w/w). Hydrogen peroxide is strong oxidant and these oxidizing properties allow it to destroy a wide range of pathogens and it is used to sterilize heat or temperature sensitive articles such as rigid endoscopes. In medical sterilization hydrogen peroxide is used at higher concentrations, ranging from around 35% up to 90%. The biggest advantage of hydrogen peroxide as a sterilant is the short cycle time. Whereas the cycle time for ethylene oxide (discussed above) may be 10 to 15 hours, the use of very high concentrations of hydrogen peroxide allows much shorter cycle times. Some hydrogen peroxide modern sterilizers, such as the Sterrad NX have a cycle time as short as 28 minutes.
[edit] Dry sterilization process
Dry sterilization process (DSP) uses hydrogen peroxide at a concentration of 30-35% under low pressure conditions. This process achieves bacterial reduction of 10−6...10−8. The complete process cycle time is just 6 seconds, and the surface temperature is increased only 10-15 °C (18 to 27 °F). Originally designed for the sterilization of plastic bottles in the beverage industry, because of the high germ reduction and the slight temperature increase the dry sterilization process is also useful for medical and pharmaceutical applications.
[edit] Peracetic acid
Peracetic acid (0.2%) is used to sterilize instruments in some STERIS Corporation systems.
[edit] Silver
Silver ions and silver compounds show a toxic effect on some bacteria, viruses, algae and fungi, typical of heavy metals like lead or mercury, but without the high toxicity to humans that is normally associated with these other metals. Its germicidal effects kill many microbial organisms in vitro, but testing and standardization of silver products is yet difficult.[26]In the antique Greek Hippocratic Corpus it is written that silver has beneficial healing and anti-disease properties[27], and the Phoenicians used to store water, wine, and vinegar in silver bottles to prevent spoiling. In the early 1900s people would put silver dollars in milk bottles to prolong the milk's freshness.[28] The exact process of silver's germicidal effect is still not well understood. One of the explanations is the oligodynamic effect, which accounts for the effect on microorganisms but not on viruses.
[edit] Radiation Sterilization
Methods of sterilization exist using radiation such as electron beams, X-rays, gamma rays, or subatomic particles.[40]
[edit] Non Ionizing Radiation Sterilization
- Ultraviolet light irradiation (UV, from a germicidal lamp) is useful only for sterilization of surfaces and some transparent objects. Many objects that are transparent to visible light absorb UV, glass for example completely absorbs all UV light. UV irradiation is routinely used to sterilize the interiors of biological safety cabinets between uses, but is ineffective in shaded areas, including areas under dirt (which may become polymerized after prolonged irradiation, so that it is very difficult to remove). It also damages some plastics, such as polystyrene foam if exposed for prolonged periods of time.
Suspension Culture
It is a type of culture in which single cell or small aggregates of cells multiply while suspended in agitated liquid medium. It is also referred to as cell cultures or cell suspension culture.
Principle:
Callus proliferates as an unorganised mass of cells. So it is very difficult to follow many cellular events during its growth and developmental phases. To overcome such limitation of callus culture, the cultivation of free cells as well as small cell aggregates in a chemically defined liquid medium as a suspension was initiated to study the morphological and biochemical changes during their growth and developmental phases. To achieve an ideal cell suspension, most commonly a friable callus is transferred to agitated liquid medium where it breaks up and disperses. After eliminating the large callus pieces, only single cells and small cell aggregates are again transferred to fresh medium and after two to three weeks a suspension of actively growing cells is produced. Ideally suspension culture should consist of only single cells which are physiologically and biochemically uniform.
The movement of nutrient medium in suspension culture provides vital aeration of the medium to sustain cell respiration in the liquid medium and also encourages the callus tissue to brake up. As the cell division starts in the callus tissue, they shed and dispense directly into medium. A more friable callus tissue is an ideal material for the dispersion of cells. Increasing the concentration of auxin or adding very low concentration of cellulose and pectinase enzymes in the liquid medium are also effective for dispersion of cells.
The period of incubation during which the suspension culture is developed from callus tissue is usually called as initiation passage. In general, media suitable for growing callus cultures for particular species are also suitable for growing suspension cultures provided that agar is omitted. The concentration of auxin and cytokinins used for callus cultures is generally reduced for suspension cultures.
Synchronization of Suspension Culture
Cells in suspension cultures vary greatly in size, shape DNA and nuclear content. Moreover, the cell cycle time varies considerably within individual cells. Therefore, cell cultures are mostly asynchromous. This variation complicates studies of biochemical, genetic physiological and other aspects of cell metabolism. A synchronous culture is one in which the majority of cells proceed through each cell cycle phase (G,S ,G2 and M) simultaneously.
A) Physical Methods:
i) Selection by Volume:
Synchronization may be achieved on the basis of selecting the size of cell aggregates present even in the finest possible suspension cultures. Cell fractionation is employed for selection.
ii) Temperature Shock:
Low temperature shocks combined with nutrient starvation are reported to induce synchronization of suspension culture.
B) Chemical Methods:
i) Starvation:
The principle of starvation is based on depriving suspension cultures of an essential growth compound leading to a stationary growth phase. Resupplying the missing compounds is expected to induce resumption of cell growth synchronously. Growth hormone starvation is also reported to induce synchronization of cell cultures.
ii) Inhibtiion:
Synchronization is achieved by temporarily blocking the progression of events in the cell cycle and accumulating cells in a specific stage using a biochemical inhibitor. On release the block cells with synchronously enter the next stage. Inhibitors of DNA synthesis ( 5-aminourail, 5-flurodexypurine, hydroxyurea or excess thymidine) in cell cultures accumulate cells at the G1/S boundary.
iii) Mitotic Arrest:
Colchicine has been widely used to arrest cells at metaphase. Suspension cultures in exponential growth are supplied with 0.02% (w/v) colchicine for 4-8 hr in order to inhibit spindle formation
Importance of Suspenion Cell Culture
1. Suspenion cultures system is capable of controlling many significant information about cell physiology, biochemistry and metabolic events at the level of individual cells and small cell aggregates.
2. By cell plating technique different cell clones can be developed.
3. Suspension cultures can be used for production of secondary metabolites.
4. Mutagenesis studies may be facilitated by the use of cell suspension culture to produce mutant cell clones from which mutant plant can be regenerated.
Callus Culture
Higher plant body is multicellular and is made up of highly organised and differentiated structures like stem, leaf, root, etc. different tissue system present in different organs function in a highly coordinated manner. Now, if the organised tissue are diverted into an unorganised proliferation mass of cell by any means, they will form the callus tissue.
In nature, sometimes callus or callus –like tissue is found to form to form in various part of intact plant either due to deep wound or due to some disease. Deep large wound in branches and trunks of intact plants results in the formation of soft mass of unorganised parenchymatous tissues which are rapidly formed on or below the injured surface of the organ concerned. Such callus tissue is known as wound callus. Wound callus is formed by the division of cambium tissues. They may also be formed by the same process from the parenchymatous cells of cortex, phloem and xylem rays. Callus like growth is also stimulated due to some disease caused by Agrobacterium tumefaciens, synchytrium endobioticum and virus, insects etc.
Such callus –like outgrowth is known as gall or tumour. But the callus in tissue culture is produce experimentally from the small excise portion called the explant of any living healthy plant. In culture, the excised plant tissue losses its structural integrity and changes completely to a rapidly proliferative unorganised mass of cells which is called the callus tissue.
Principles of Callus Culture
For successful initiation of callus, three important criteria should be accomplished.
i) Aseptic preparation of plant material.
ii) Selection of suitable nutrient medium supplemented with appropriate ration of auxin and cytokinins or only appropriate auxin, and
iii) Incubation of culture under controlled physical condition.
ii) Selection of suitable nutrient medium supplemented with appropriate ration of auxin and cytokinins or only appropriate auxin, and
iii) Incubation of culture under controlled physical condition.
Different plant parts carry a number of surface borne micro-organism like bacteria, fungus, etc. The excised plant parts called explants are at first washed with liquid detergent. Then explants are surface sterilized by the most commonly used chemical such as 0.1% w/v mercuric chloride (Hgcl2) or sodium hypochloride (0.8% to 1.6% available chloride) for a limited time ( generally 10-15 minutes). After sterilization, the explants are repeatedly rinsed with autoclaved distilled water.
The surface sterilized plant material is cut aseptically into small segments (a few millimetres in size) and are transferred aseptically on a suitable nutrient medium solidified with agar.
Agar solidified or semi-solid nutrient medium after its preparation and sterilization by autoclave at 15 lbs, pressure for 15 minutes is used for induction of callus tissue. For most successful callus culture and for healthy callus growth usually both an auxin and cytokinins are required.
The suitable temperature for in vitro callus initiation and growth is usually 25+- 2 0C. In some plant materials initiation and growth of callus take place in totally dark condition. However, in some cases a particular photoperiod (16 hrs light and 8hrs dark) is necessary for initiation and growth of callus tissues. Approximately, 2000 to 3000 lux artificial light intensity is needed. Generally, 55 to 60 % relative humidity is maintained in culture room.
Once the growth of the callus tissue is well established, portions of the callus tissue can be removed and transferred directly on to fresh nutrient medium to continue growth. In this manner, callus cultures can be continuously maintained by serial subcultures.
Application of Callus Culture
1. The whole plant can be regenerated in large number from callus tissue through manipulation of the nutrient and hormonal constituents in the culture medium which is called as organogenesis or morphogenesis. Similarly, callus can be induce to form somatic embryo which can gives rise to whole plant.
2. Callus tissue is good source of genetic or karyotypic variability, so it may be possible to regenerate a plant from genetically variable cells of the callus tissue.
3. Cell suspension culture in moving liquid medium can be initiated from callus culture.
4. Callus culture is very useful to obtain commercially important secondary metabolites. If a bit tissue from a medicinally important plant is grown in vitro and produced callus culture, then secondary metabolites or drugs can be directly extracted from the callus tissues without sacrfting the whole plant.
5. Several biochemical assays can be performed from callus culture.
Male Sterility
Male sterility is defined as an absence or non-function of pollen grain in plant or incapability of plants to produce or release functional pollen grains. The use of male sterility in hybrid seed production has a great importance as it eliminate the process of mechanical emasculation.
Features of Male Sterility
- Prevents self pollination, permits cross pollination.
- Leads to heterozygosity
- Female gametes function normally
- Assayed through staining techniques
- In nature, occur due to spontaneous mutations
- Can be induced artificially
Types of Male Sterility
Male sterility can be of following types
- Cytoplasmic male sterility (CMS) – governed by cytoplasmic genes
- Genetic male sterility (GMS) – governed by nuclear genes
- Cytoplasmic-Genetic male sterility (CGMS) – governed by both nuclear and cytoplasmic genes
- Transgenic male sterility – induced by the technique of genetic engineering
- Chemical induced male sterility – induced by the use of chemical
Limitations of Male Sterility system from the Plant breeding point of view
- In some crops, sterile cytoplasm has adverse effect on yield.
- Unsatisfactory restoration of fertility
- Break down of male sterility because of some reasons like, certain environmental conditions which leads to some pollen production by the male sterile lines and cytoplasm contribution (though small) by the sperm in some cases,
- Difficult to identify line with GMS
- Unsatisfactory or poor pollination
- Modifiers or modifying genes may affect cytoplasmic male sterility
VIRUS RESISTANCE PLANTS
Plants can suffer from infections caused by fungi, bacteria, viruses, nematodes, and other pathogens. Various high-tech approaches have been proposed to protect plants from harmful afflictions. To date, most interest has been focused on virus resistant transgenic plants, but using biotechnology to confer resistance to fungi, bacteria, or nematodes has also been gaining attention.
Viral diseases
Viruses cause many economically important plant diseases. For example, the Beet necrotic yellow vein virus (BNYVV) causes sugar beets to have smaller, hairier roots, reducing yields by up to 50 percent. The spread of most viruses is very difficult to control. Once infection sets in, no chemical treatment methods are available. Losses are usually very high and require longer rotation intervals and modified cropping systems. This translates into considerable losses.
Viruses are often transmitted from plant to plant by insects. Insecticides are sometimes used to control viral infections, but success is very limited.
|
The most effective ways of managing viruses are cultural controls (e.g. removing diseased plants) and using resistant cultivars. Although conventional methods of breeding have been able to provide some  virus resistant or tolerant cultivars, they are not available for most corps.
virus resistant or tolerant cultivars, they are not available for most corps.
Virus resistant GM plants
In some cases, biotechnology can be used to make virus resistant crops. The most common way of doing this is by giving a plant a viral gene encoding the virus' 'coat protein'. The plant can then produce this viral protein before the virus infects the plant. If the virus arrives, it is not able to reproduce.
The explanation for this is called cosuppression. The plant has ways of knowing that the viral coat protein should not be produced, and it has ways of eventually shutting down the protein's expression. When the virus tries to infect the plant, the production of its essential coat protein is already blocked.
All  genetically modified virus resistant plants on the market (e.g. papayas and squash) have coat protein mediated resistance. It may also be possible to confer resistance by taking a resistance gene naturally found in one plant and then transferring it to an important crop.
genetically modified virus resistant plants on the market (e.g. papayas and squash) have coat protein mediated resistance. It may also be possible to confer resistance by taking a resistance gene naturally found in one plant and then transferring it to an important crop.
INSECT RESISTANCE
Insect attack is a serious agricultural problem leading to yield losses and reduced product quality. Insects can cause damage both in the field and during storage in silos. Each year, insects destroy about 25 percent of food crops worldwide. The larvae of Ostrinia nubilalis, the European corn borer, can destroy up to 20 percent of a maize crop.
The “Bt concept” – pest resistant transgenic plants
Bacillus thuringiensis, or
When consumed by insects, the protein is converted to its active, toxic form (delta endotoxin), which in turn destroys the gut of the insect. Bt preparations are commonly used in organic agriculture to control insects, as Bt toxin occurs naturally and is completely safe for humans.
More than 100 different variations of Bt toxin have been identified in diverse strains of Bacillus thuringiensis. The different variations have different target insect specificity. For example, the toxins classified under Cry1a group target Lepidoptera (butterflies), while toxins in the Cry3 group are effective against beetles.
Researchers have used genetic engineering to take the bacterial genes needed to produce Bt toxins and introduce them into plants. If plants produce Bt toxin on their own, they can defend themselves against specific types of insects. This means farmers no longer have to use chemical insecticides to control certain insect problems.
|
Critics claim that in some cases the use of insect resistant crops can harm beneficial insects and other non-target organisms. Extensive ecological impact assessments have been addressing these issues. In the field, no significant adverse effects on non-target wildlife nor long term effects of higher Bt concentrations in soil have yet been observed.
New concepts on the way
Bt crops have been planted commercially for more than eight years. Other naturally occuring insecticidal compounds are now becoming available as alternatives to the Bt approach. Among these are  chitinase, lectins, alpha-amylase inhibitors, proteinase inhibitors, and cystatin. Plants genetically modified to express these defense proteins are still in early stages of development.
chitinase, lectins, alpha-amylase inhibitors, proteinase inhibitors, and cystatin. Plants genetically modified to express these defense proteins are still in early stages of development.
Anther or Pollen culture
Pollen culture (microspore culture) is a technique in which haploid plants are obtained from isolated pollen grains while in anther culture those are obtained from pollens, by placing anthers on a suitable, synthetic culture medium.
The technique was discovered by Guha and Maheshwari (1964). It is one of the various tissue culture techniques or methods used. Progeny developed by this technique contains a single set of chromosomes.
Importance:
- the technique is fairly simple
- it is easy to induce cell division in the immature pollen cells in some species
- a large proportion of the anthers used in culture respond (induction frequency is high)
▪ Haploids can be produced in large numbers very quickly
Some disadvantages of using anther culture to obtain haploids are:
- when working with some species, the majority of plants produced have been non-haploid
- in cereals, very few green plants are obtained; many of the plants are albinos or green-albino chimeras
- it is tedious to remove the anthers without causing damage
- sometimes a particular orientation is necessary to acheive a desired responce
Procedure:
Young flower bud with immature anther at the appropriate stage of pollen development is surface sterilized & rinsed with sterile water. The buds are dissected by removing the sepals & petals. The anthers are carefully dissected from the flower buds & kept in a petridish. Each anther is gently separated from the filament & the intact uninjured anthers are placed horizontally on nutrient medium. If the flowers are very small a sterio microsecope can be used for dissecting the anthers. Isolated healthy anthers are cultured on agar medium or in a liquid medium. The anther burst open within 3-8 weeks of culture. After they have attained a height of about 3-5 cm the individual plantlets or shoots emerging from the callus are separated & transferred to a medium that would support further development. The rooted plants are transferred to soil mix in the pots.
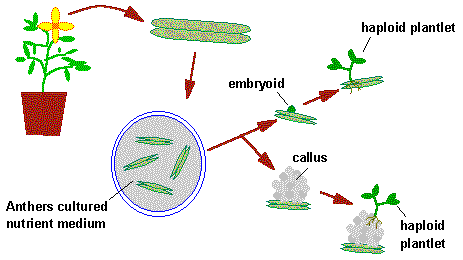
Application of Haploids in agriculture:
1. Reduction of time in developing variety of self pollinated crop:
2. Production of pure line of self-incompatible species
3. Hybrid production in crop
4. Study of mutation
5. Induction of genetic variability
6. Fusion of protoplast of haploid cell
7. Cytogenetic research:
8. Allow breeding at the haploid level;
9. Development of super males in Asparagus
10. Selection of resistant lines
Limitations
- The application of this technique is not yet possible in many crops where proper protocol is not yet developed.
- High level of management & expertise are required to operate the tissue culture production of haploid.
- Mixture of different ploidy levels makes the task difficult.
- Relatively high incidence of albinism in some types of anther & pollen culture is defected.
- The lack of selection of traits during the derivation of haploid material.
- In some cases, the doubling of a haploid does not always result in the production of a homozygote.
Ti Plasmid
Agrobacterium tumefaciens is a rod shaped Gram negative soil bacteria that has a 200kb plasmid called as Ti plasmid (tumor inducing plasmid) which is responsible for crown gall disease.
- is a large 200 kb plasmid
- It has 4 major regions
- T-DNA region or transfer DNA region which has genes for auxin, cytokinin and opine
- T-DNA region is integrated into the plant genome
- A left border and right border repeats
- An origin of replication (ori)
- Virulence region that has genes that mediate T DNA transfer
- Opine catabolism region that has genes for opine utilization
T-DNA region
T-DNA or transfer DNA is the region of Ti plasmid that is transferred and integrated into the plant genome.
- Auxin and cytokinin gene induces cell division and proliferation leading to the crown gall disease.
- Opine gene will synthesize opines like octopine, noopaline etc These are nutrients for the growth of bacteria.
- LB and RB are required for transfer.
RI Plasmid
Agrobacterium rhizhogenes is a Gram negative soil bacterium that incites hairy root disease in dicot plants. A plasmid called Ri plasmid (root hair inducing plasmid) is responsible for this disease.
Ri plasmids are analogous to Agrobacterium tumefaciens Ti plasmid.
Ri plasmid organization:
- Large plasmid of 250kb
- Has 2 T-DNA fragments Tr-DNA and Tl-DNA (left and right) separated by 15 kB segment
- Tr-DNA is similar to T-DNA. It has genes for auxin and agropine synthesis
(a type of opine) - Tl-DNA has 4 genes: rolA, rolB, rolC and rolD (rol=root loci)
- Rol A: responsible for hairy root formation
- Rol B: induces root initiation and callus formation
- Rol C: promotes root growth
- Rol D: suppresses callus growth
- Then Ri plasmid has a virulence region that mediated T DNA transfer and an ori same as Ti plasmid.
Uses
- Ri plasmids can be used as substitutes for Ti plasmids to transfer foreign genes to plants
- Ri plasmids exchange DNA segments between plants and bacteria and hence they could be study gene exchange between the organisms.
- They could be used to induce rooting in clones where rooting from callus or explant is difficult.
- They could also be used to increase nodulation frequency of plantlets during micropropagation.