Feedback inhibition
Feedback inhibition, in enzymology, suppression of the activity of an enzyme, participating in a sequence of reactions by which a substance is synthesized, by a product of that sequence. When the product accumulates in a cell beyond an optimal amount, its production is decreased by inhibition of an enzyme involved in its synthesis. After the product has been utilized or broken down and its concentration thus decreased, the inhibition is relaxed, and the formation of the product resumes. Such enzymes, whose ability to catalyze a reaction depends upon molecules other than their substrates (the ones upon which they act to form a product), are said to be under allosteric control. Feedback inhibition is a mechanism by which the concentration of certain cell constituents is limited.
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H (also written as CH3COOH or C2H4O2). It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar (apart from water; vinegar is roughly 8% acetic acid by volume), and has a distinctive sour taste and pungent smell. Besides its production as household vinegar, it is mainly produced as a precursor to polyvinylacetate and cellulose acetate. Although it is classified as a weak acid, concentrated acetic acid is corrosive, and attacks the skin.
Oxidative fermentation
For most of human history, acetic acid bacteria of the genus Acetobacter have made acetic acid, in the form of vinegar. Given sufficient oxygen, these bacteria can produce vinegar from a variety of alcoholic foodstuffs. Commonly used feeds include apple cider, wine, and fermented grain, malt, rice, or potato mashes. The overall chemical reaction facilitated by these bacteria is:
C2H5OH + O2 → CH3COOH + H2O
A dilute alcohol solution inoculated with Acetobacter and kept in a warm, airy place will become vinegar over the course of a few months. Industrial vinegar-making methods accelerate this process by improving the supply of oxygen to the bacteria.The first batches of vinegar produced by fermentation probably followed errors in the winemaking process. If must is fermented at too high a temperature, acetobacter will overwhelm the yeast naturally occurring on the grapes. As the demand for vinegar for culinary, medical, and sanitary purposes increased, vintners quickly learned to use other organic materials to produce vinegar in the hot summer months before the grapes were ripe and ready for processing into wine. This method was slow, however, and not always successful, as the vintners did not understand the process.[29]
One of the first modern commercial processes was the "fast method" or "German method", first practised in Germany in 1823. In this process, fermentation takes place in a tower packed with wood shavings or charcoal. The alcohol-containing feed is trickled into the top of the tower, and fresh air supplied from the bottom by either natural or forced convection. The improved air supply in this process cut the time to prepare vinegar from months to weeks.[30]
Nowadays, most vinegar is made in submerged tank culture, first described in 1949 by Otto Hromatka and Heinrich Ebner.[31] In this method, alcohol is fermented to vinegar in a continuously stirred tank, and oxygen is supplied by bubbling air through the solution. Using modern applications of this method, vinegar of 15% acetic acid can be prepared in only 24 hours in batch process, even 20% in 60-hour fed-batch process.[29]
Anaerobic fermentation
Species of anaerobic bacteria, including members of the genus Clostridium or Acetobacterium can convert sugars to acetic acid directly, without using ethanol as an intermediate. The overall chemical reaction conducted by these bacteria may be represented as:
C6H12O6 → 3 CH3COOH
These acetogenic bacteria produce acetic acid from one-carbon compounds, including methanol, carbon monoxide, or a mixture of carbon dioxide and hydrogen:
2 CO2 + 4 H2 → CH3COOH + 2 H2O
This ability of Clostridium to utilize sugars directly, or to produce acetic acid from less costly inputs, means that these bacteria could potentially produce acetic acid more efficiently than ethanol-oxidizers like Acetobacter. However, Clostridium bacteria are less acid-tolerant than Acetobacter. Even the most acid-tolerant Clostridium strains can produce vinegar of only a few per cent acetic acid, compared to Acetobacter strains that can produce vinegar of up to 20% acetic acid. At present, it remains more cost-effective to produce vinegar using Acetobacter than to produce it using Clostridium and then concentrate it. As a result, although acetogenic bacteria have been known since 1940, their industrial use remains confined to a few niche applications.Uses
Acetic acid is a chemical reagent for the production of chemical compounds. The largest single use of acetic acid is in the production of vinyl acetate monomer, closely followed by acetic anhydride and ester production. The volume of acetic acid used in vinegar is comparatively small.[24]
Vinyl acetate monomer
The major use of acetic acid is for the production of vinyl acetate monomer (VAM). This application consumes approximately 40% to 45% of the world's production of acetic acid.
Vinyl acetate can be polymerized to polyvinyl acetate or to other polymers, which are components in paints and adhesives.Ester production
The major esters of acetic acid are commonly used solvents for inks, paints and coatings.Acetic anhydride
The product of the condensation of two molecules of acetic acid is acetic anhydride.
Acetic anhydride is an acetylation agent. As such, its major application is for cellulose acetate, a synthetic textile also used for photographic film. Acetic anhydride is also a reagent for the production of heroin and other compounds.Vinegar
Vinegar is typically 4-18% acetic acid by mass. Vinegar is used directly as a condiment, and in the pickling of vegetables and other foods.Use as solvent
Glacial acetic acid is an excellent polar protic solvent, as noted above. It is frequently used as a solvent for recrystallization to purify organic compounds. Acetic acid is used as a solvent in the production of terephthalic acid (TPA), the raw material for polyethylene terephthalate (PET). In 2006, about 20% of acetic acid is used for TPA production.[24]
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. A psychoactive drug and one of the oldest recreational drugs known, ethanol produces a state known as alcohol intoxication when consumed. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a solvent, and as a fuel. In common usage, it is often referred to simply as alcohol or spirits.
Fermentation
Ethanol for use in alcoholic beverages, and the vast majority of ethanol for use as fuel,[citation needed] is produced by fermentation. When certain species of yeast (e.g., Saccharomyces cerevisiae) metabolize sugar in reduced-oxygen conditions they produce ethanol and carbon dioxide. The chemical equations below summarize the conversion:
C6H12O6 → 2 CH3CH2OH + 2 CO2
C12H22O11 + H2O → 4 CH3CH2OH + 4 CO2
Fermentation is the process of culturing yeast under favorable thermal conditions to produce alcohol. This process is carried out at around 35–40 °C. Toxicity of ethanol to yeast limits the ethanol concentration obtainable by brewing; higher concentrations, therefore, are usually obtained by fortification or distillation. The most ethanol-tolerant strains of yeast can survive up to approximately 15% ethanol by volume.[44]To produce ethanol from starchy materials such as cereal grains, the starch must first be converted into sugars. In brewing beer, this has traditionally been accomplished by allowing the grain to germinate, or malt, which produces the enzyme amylase. When the malted grain is mashed, the amylase converts the remaining starches into sugars. For fuel ethanol, the hydrolysis of starch into glucose can be accomplished more rapidly by treatment with dilute sulfuric acid, fungally produced amylase, or some combination of the two.
Uses
As a fuel
The largest single use of ethanol is as a motor fuel and fuel additive.
Ethanol may also be utilized as a rocket fuel, and is currently in lightweight rocket-powered racing aircraft.Alcoholic beverages
Ethanol is the principal psychoactive constituent in alcoholic beverages, with depressant effects on the central nervous system.Feedstock
Ethanol is an important industrial ingredient and has widespread use as a base chemical for other organic compounds. These include ethyl halides, ethyl esters, diethyl ether, acetic acid, ethyl amines, and, to a lesser extent, butadiene.Antiseptic
Ethanol is used in medical wipes and in most common antibacterial hand sanitizer gels at a concentration of about 62% v/v as an antiseptic. Ethanol kills organisms by denaturing their proteins and dissolving their lipids and is effective against most bacteria and fungi, and many viruses, but is ineffective against bacterial spores.[80]Treatment for poisoning by other alcohols
Ethanol is sometimes used to treat poisoning by other, more toxic alcohols, in particular methanol[81] and ethylene glycol.Solvent
Ethanol is miscible with water and is a good general purpose solvent. It is found in paints, tinctures, markers, and personal care products such as perfumes and deodorants. It may also be used as a solvent or solute in cooking, such as in vodka sauce.
Antibiotics
Antibiotics are substances that inhibit the growth of or destroy bacteria that cause infection. Antibiotics do not work against viral diseases such as thecommon cold or influenza. The word "antibiotics" comes from the Greek anti("against") and bios("life"). Antibiotics have been used since the 1930s to prevent or treat a wide variety of infections in plants, animals,and humans. Before that time, there were few effective ways of combating microbial infections (infections caused by microorganisms). Illnesses such as pneumonia, tuberculosis, and typhoid fever were essentially untreatable. Even minor infections could be deadly.
The overwhelming majority of antibiotics are made from living organisms suchas bacteria about 90% of antibiotics are isolated from bacteria fungi, and molds. Others are produced synthetically, either in whole or in part.
At one time all antibiotics were made from living organisms. This process, known as biosynthesis, is still used in the manufacture of a number of antibiotics. In this method, it is actually the organisms themselves that manufacturethe antibiotic. The laboratory technician merely provides favorable conditions for the organisms to multiply, and then extracts the drug. For example, mold organisms are placed in a medium (a substance used for the growth of microorganisms) such as corn liquor to which milk sugar has been added. This mixture forms a liquid that is put into a tank, which is kept at a temperature of25 degrees Centigrade (77 degrees Fahrenheit) and shaken for over 100 hours.The mold organisms multiply rapidly in this warm liquid, producing penicillinin the process.
All types of penicillin have an identical ring. However, in each type of penicillin, the chemical chain attached to the ring is different. By modifying the molecules of the chain, scientists are able to create drugs with a wide range of effects on a variety of organisms. Some of these drugs are useful in treating infections.
Pharmaceutical companies use computer-generated images of the rings and experiment with a countless variety of possible chains. Researchers have developedantibiotics with long half-lives (period of effectiveness), which means thatthe medication can be taken every 24 hours instead of every few hours. The newer antibiotics are also more effective against a wider range of infectionsthan were earlier drugs.
USES OF BROAD SPECTRUM ANTIBIOTICS:
Broad-spectrum antibiotics are properly used in the following medical situations:
Broad-spectrum antibiotics are properly used in the following medical situations:
- Empirically prior to identifying the causative bacteria when there is a wide differential and potentially serious illness would result in delay of treatment. This occurs, for example, in meningitis, where the patient can become so ill that he/she could die within hours if broad-spectrum antibiotics are not initiated.
- For drug resistant bacteria that do not respond to other, more narrow spectrum antibiotics.
- In super-infections where there are multiple types of bacteria causing illness, thus warranting either a broad-spectrum antibiotic or combination antibiotic therapy.
ADVANTAGES OF BROAD SPECTRUM ANTIBIOTICS:
- Broader Spectra of Activity
- A clear advantage to the use of broad-spectrum antibiotics is that there is less of a need (as compared with narrow-spectrum antibiotics) to identify the infecting pathogen with real certainty before commencing treatment.
DISADVANTAGES OF BROAD SPECTRUM ANTIBIOTICS:
- Children who receive broad-spectrum antibiotics during their first year of life are at increased risk of developing childhood asthma.
- Broad Spectrum antibiotics may give rise to drug resistance.
BROAD SPECTRUM ANTIBIOTICS:
- Amoxicillin
- Levofloxacin
- Gatifloxacillin
- Streptomycin
- Tetracycline
- Chloramphenicol
Xanthan gum
Xanthan gum is a polysaccharide secreted by the bacterium Xanthomonas campestris [2], used as a food additive and rheology modifier,[3] commonly used as a food thickening agent (in salad dressings, for example) and a stabilizer (in cosmetic products, for example, to prevent ingredients from separating). It is produced by the fermentation of glucose, sucrose, or lactose. After a fermentation period, the polysaccharide is precipitated from a growth medium with isopropyl alcohol, dried, and ground into a fine powder. Later, it is added to a liquid medium to form the gum.[4]
Preparation
The polysaccharide is prepared by inoculating a sterile aqueous solution of carbohydrate(s), a source of nitrogen, dipotassium phosphate, and some trace elements. The medium is well-aerated and stirred, and the polymer is produced extracellularly into the medium. The final concentration of xanthan produced will vary greatly depending on the method of production, strain of bacteria, and random variation.They grew the bacteria in a yeast broth and placed it in an incubator, which measured the 4 degrees Celcius (39.2 degrees Fahrenheit) temperature constantly to insure optimum bacteria growth. Then they added sugar-beet molasses to the flasks of bacteria. The flasks were shaken constantly for five days, using a water-bath shaker. The researchers added ethanol to recover xanthan, then dried the remains of the flasks for 24 to 48 hours to recover xanthan gum.
Uses
One of the most remarkable properties of xanthan gum is its ability to produce a large increase in the viscosity of a liquid by adding a very small quantity of gum, on the order of one percent.
In foods, xanthan gum is most often found in salad dressings and sauces. It helps to prevent oil separation by stabilizing the emulsion, although it is not an emulsifier. In the oil industry, xanthan gum is used in large quantities, usually to thicken drilling mud.
In cosmetics, xanthan gum is used to prepare water gels, usually in conjunction with bentonite clays. It is also used in oil-in-water emulsions to help stabilize the oil droplets against coalescence. It has some skin hydrating properties. Xanthan gum is a common ingredient in fake blood recipes, and in gunge/slime.
Dextrin
Dextrins are a group of low-molecular-weight carbohydrates produced by the hydrolysis of starch[1] or glycogen.[2] Dextrins are mixtures of polymers of D-glucose units linked by α-(1→4) or α-(1→6) glycosidic bonds.
Dextrins can be produced from starch using enzymes like amylases, as during digestion in the human body and during malting and mashing,[3] or by applying dry heat under acidic conditions (pyrolysis or roasting). The latter process is used industrially, and also occurs on the surface of bread during the baking process, contributing to flavor, color, and crispness. Dextrins produced by heat are also known as pyrodextrins. During roasting under acid condition the starch hydrolyses and short chained starch parts partially rebranch with α-(1,6) bonds to the degraded starch molecule.[4]
Dextrins are white, yellow, or brown powders that are partially or fully water-soluble, yielding optically active solutions of low viscosity. Most can be detected with iodine solution, giving a red coloration; one distinguishes erythrodextrin (dextrin that colours red) and achrodextrin (giving no colour).
White and yellow dextrins from starch roasted with little or no acid is called British gum.
Uses
Yellow dextrins are used as water-soluble glues [5] in remoistable envelope adhesives and paper tubes, in the mining industry as additives in froth flotation, in the foundry industry as green strength additives in sand casting, as printing thickener for batik resist dyeing, and as binders in gouache paint.White dextrins are used as:
- a crispness enhancer for food processing, in food batters, coatings, and glazes, (E number 1400)
- a textile finishing and coating agent to increase weight and stiffness of textile fabrics
- a thickening and binding agent in pharmaceuticals and paper coatings.
Due to the rebranching, dextrins are less digestible; indigestible dextrin are developed as soluble fiber supplements for food products
Biopesticide
Biopesticides, a contraction of 'biological pesticides', include several types of pest management intervention: through predatory, parasitic, or chemical relationships. The term has been associated historically with biological control - and by implication - the manipulation of living organisms.
Biopesticides fall into three major classes:
- Microbial pesticides which consist of bacteria, entomopathogenic fungi or viruses (and sometimes includes the metabolites that bacteria or fungi produce). Entomopathogenic nematodes are also often classed as microbial pesticides, even though they are multi-cellular.
- Biochemical pesticides are naturally occurring substances that control (or monitor in the case of pheromones) pests, but are relatively non-toxic to mammals.
- Plant-incorporated protectants (PIPs) have genetic material from other species incorporated into their genetic material (i.e. GM crops): still controversial in some (notably European) countries.
Applications
Biopesticides are typically microbial biological pest control agents that are applied in a manner similar to chemical pesticides. In order to implement these environmentally friendly pest control agents effectively, it can be important to pay attention to the way they are formulated[5] and applied.[6]Biopesticides for use against crop diseases have already established themselves on a variety of crops. For example, biopesticides already play an important role in controlling downy mildew diseases.
A major growth area for biopesticides is in the area of seed treatments and soil amendments. Fungicidal and biofungicidal seed treatments are used to control soil borne fungal pathogens that cause seed rots, damping-off, root rot and seedling blights. They can also be used to control internal seed–borne fungal pathogens as well as fungal pathogens that are on the surface of the seed. Many biofungicidal products also show capacities to stimulate plant host defenses and other physiological processes that can make treated crops more resistant to a variety of biotic and abiotic stresses.
Advantages
- Harmful residues not detected
- Can be cheaper than chemical pesticides when locally produced.
- Can be more effective than chemical pesticides in the long-term (as demonstrated, for example, by the LUBILOSA Programme)
Disadvantages
- High specificity: which may require an exact identification of the pest/pathogen and the use of multiple products to be used
- Often slow speed of action (thus making them unsuitable if a pest outbreak is an immediate threat to a crop)
- Often variable efficacy due to the influences of various biotic and abiotic factors (since biopesticides are usually living organisms, which bring about pest/pathogen control by multiplying within the target insect pest/pathogen)
- Living organisms evolve and increase their resistance to biological, chemical, physical or any other form of control. If the target population is not exterminated or rendered incapable of reproduction, the surviving population can acquire a tolerance of whatever pressures are brought to bear, resulting in an evolutionary arms race.
Single Cell Protein
The term Single Cell Protein (SCP) refers to the dried microbial cells or total protein extracted from pure microbial cell culture (Algae, bacteria, filamentous fungi, yeasts), which can be used as food supplement to humans (Food Grade) or animals (Feed grade). Most of the developing countries of the world are facing a major problem of malnutrition. Due to rapid growth in the population deficiency of protein and nutrients are seen in human food and as well as animal feed. Single cell proteins have application in animal nutrition as: fattening calves, poultry, pigs and fish breading. In food it is used as : aroma carriers, vitamin carrier, emulsifying aids and to improve the nutritive value of baked products, in soups, in ready-to-serve-meals, in diet recipes and in the technical field in : paper processing, leather processing and as foam stabilizers.Protein (SCP) refers to the dried microbial cells or total
Production of Single Cell Protein
The production of Single Cell Protein can be done by using waste materials as the substrate, specifically agricultural wastes such as wood shavings, sawdust, corn cobs, and many others. Examples of other waste material substrates are food processing wastes, residues from alcohol production, hydrocarbons, or human and animal excreta.
The process of SCP production from any microorganism or substrate would have the following basic steps:
The process of SCP production from any microorganism or substrate would have the following basic steps:
- Provision of a carbon source; it may need physical and/or chemical pretreatments. Addition, to the carbon source, of sources of nitrogen, phosphorus and other nutrients needed to support optimal growth of the selected microorganism.
- Prevention of contamination by maintaining sterile or hygienic conditions. The medium components may be heated or sterilized by filtration and fermentation equipments may be sterilized.
- The selected microorganism is inoculated in a pure state.
- SCP processes are highly aerobic (except those using algae). Therefore, adequate aeration must be provided. In addition, cooling is necessary as considerable heat is generated.
- The microbial biomass is recovered from the medium.
- Processing of the biomass for enhancing its usefulness and/or storability.
Advantages and Disadvantages of Single Cell Protein
Large scale SCP production has some advantages over the conventional food production, these advantages are :
- Microorganisms have a high rate of multiplication to hence rapid succession of generation (algae: 2-6hours, yeast: 1-3 hours, bacteria: 0.5-2 hours)
- They can be easily genetically modified for varying the amino acid composition.
- A very high protein content 43-85 % in the dry mass.
- They can utilize a broad spectrum of raw materials as carbon sources, which include even waste products. Thus they help in the removal of pollutants also.
- Strains with high yield and good composition can be selected or produce relatively easily.
- Microbial biomass production occurs in continuous cultures and the quality is consistent since the growth is independent of seasonal and climatic variations.
- Land requirements is low and is ecologically beneficial.
- It is not dependent on climate
Crab free effect
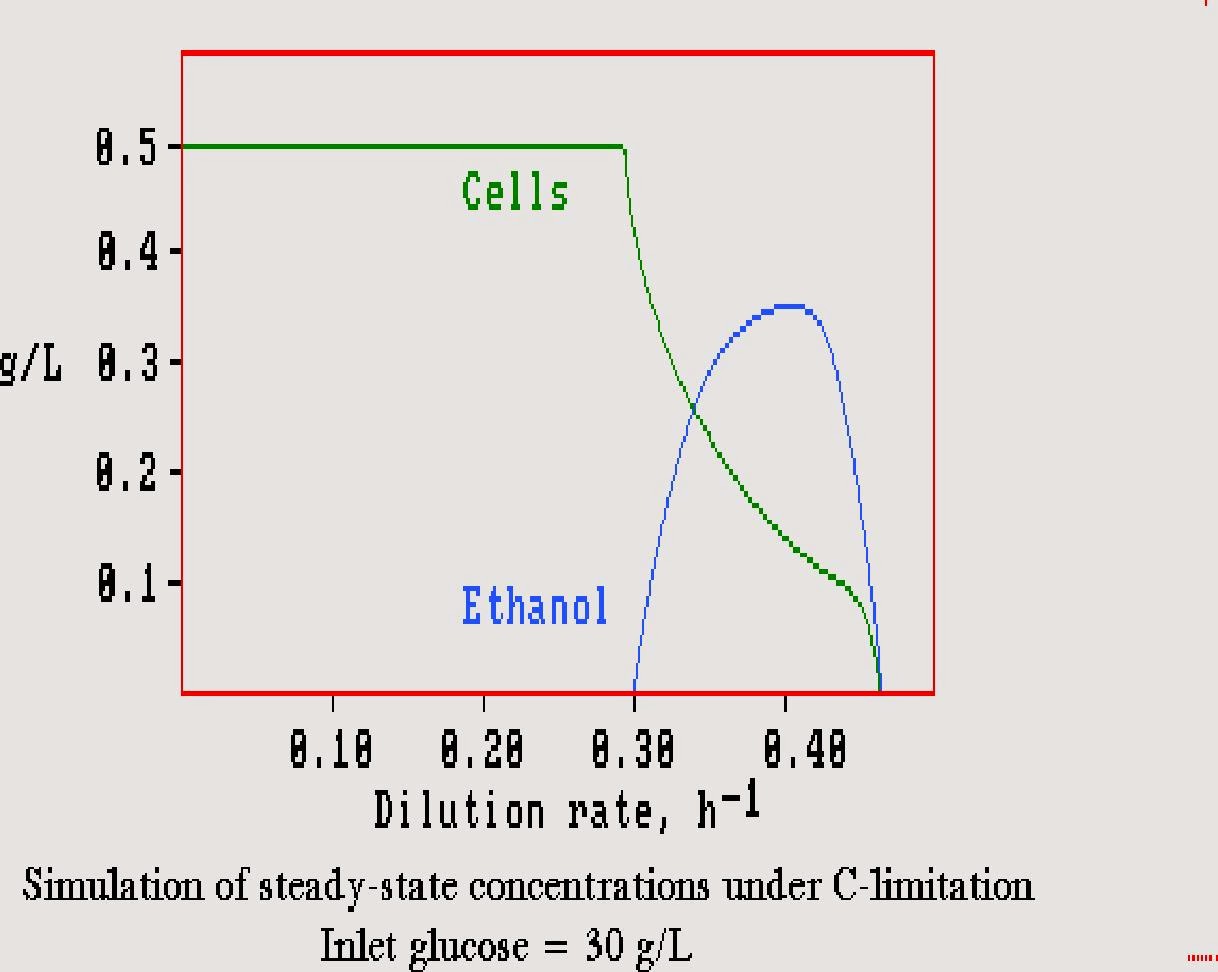
Fermentation of sugars to ethanol is an anaerobic process. However, ethanol has been found in aerated processes when the concentration of sugar is high. This is the "Crabtree effect" and was for many years considered catabolite repression. In other words, it was postulated that excess glucose repressed its use in the pathways that lead to ethanol. Fairly recently it has been shown that this hypothesis is false, and the effect seems to be over saturation of the respiratory pathways. The yeast cannot pass the excess sugar through the main path and simply shunt it through an alternate route to ethanol.
The observations in continuous culture with aeration and ethanol are that dilution rates that are far from the growth rate maximum result in quite low sugar concentrations and no overload of respiratory capacity. As we have seen, the sugar concentration rises abruptly and steeply when approaching the maximum specific growth rate, and this results in the formation of ethanol. This is seen in the following figure drawn by computer simulation:It is practically impossible to operate a chemostat near the maximum specific growth rate because this is a high-gain region for control. However, an auxostat works just fine in this region. There is potential for a rugged aerobic process that produces ethanol at high rates in an auxostat. Unfortunately, our group found that accumulation of ethanol diminishes the effect of over saturation. Perhaps genetic engineering can overcome this restriction.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.